To change the symbol of an atom double-click. The allene molecule has the following Lewis structure.

How To Draw The Mg2 Lewis Dot Structure Youtube
Mg2 Draw the Lewis dot structure for Mg2.

. While drawing a Lewis structure the following steps must be followed. Formation Magnesium oxide MgO by the transfer of electrons. Show the formal charges of all atoms in the correct structure.
Draw the Lewis dot structure for Se2. Draw the lewis dot structure for the atom and the ion. Draw the Lewis electron dot diagram for each ion.
Who are the experts. You can tell the number of valence electrons from the group number. One magnesium atom loses two electrons to become a 2 ion cationTwo chlorine atoms gain one electron each to become two -1 ions anionsThese are held t.
Try to arrange the atoms to yield the most typical number of bonds for each atom. Show the formal charge of the atom. Are all four hydrogen atoms in the same plane.
Draw the Lewis dot structure for Mg2. The Lewis dot structure for Magnesium is an Mg with 2 dots which stand for its two valence electrons. Draw the Lewis dot structure for Mg2.
Drawing Lewis Structures 2 Step 2Draw a reasonable skeletal structure using single bonds to join all the atoms. Draw the lewis dot structure for mg2 Coffin nails are getting to be the most popular manicure development today and that is going nowhere quicklyBlack appears to be fantastic with any colorNevertheless it doesnt help it become any considerably less appealing. The Lewis dot structure for Sulfur is an S with 6.
Draw Electron Dot Structure For The Formation Of Magnesium Oxide. Draw the Lewis electron dot diagram for each ion. Also does it have a total of 4 bonds.
When magnesium reacts with oxygen the magnesium atom transfers its two outermost. Magnesium Oxide MgO is the most chemically active element. Draw and explain the Lewis structure for the Mg2 ion.
Individual valencies of each atom are added up to calculate the total amount of valence electrons present in the molecule. Show the formal charges of all atoms in the correct structure. The Lewis dot structure for Magnesium is an Mg with 2 dots which stand for its two valence electrons.
Draw the lewis dot structure for the atom and the ion. We review their content and use your feedback to keep the quality high. Experts are tested by Chegg as specialists in their subject area.
Draw the lewis structure for. Take one valence electron out of the antibonding σ 3s and we have a bond order that changed from 1 2 2 2 0 to 1 2 2 1 1 2. Write lewis dot structure of Mg2 - 10830739.
The rest of the valence electrons are. To change the symbol of an atom double-click on the atom and enter the letter of the new atom. These two elements when bonded together form an ionic bond as the Magnesium loses its two valence electrons to.
To change the symbol of an atom double-click on the atom and enter the letter of the new atom. How to write lewis dot structures electron dot structures do now. By signing up youll get thousands of step-by-step solutions to your homework.
Read both sides of the handout. For the Mg2 structure use the periodic table to find the total number of valence electrons for Mg. H- If so that is the Lewis dot structure for the hydride ion.
As added electrons equal the magnitude of negative charges. Correct answer to the question Draw the Lewis dot structure for Mg2. For the lewis structure of BCl3 does it have a total of 24 valence electrons.
What is the lewis dot structure of C2H5F. The Lewis Dot Structure for MgBr2 Magnesium Bromide is as follows. Its atomic number is 12 placed in 2nd group 2 and 3rd period in the modern periodic table.
The allene molecule has the following Lewis structure. To change the symbol of an atom double-click on the atom and enter the letter of the new atom. Thus MO theory suggests that Mg 2 is more stable than Mg2.
Draw The Lewis Dot Structure For Mg2. Extra electrons are added to the lewis dot structure of the molecule if it appears to be an anion. Since MgO is an ionic compound we write the Lewis dot structure as -Mg2O2- you undestand what i mean right sorry i dont have gifjust give all the eight e- to O atom and make the.
Once we know how many valence electrons there are in Magn. Valence Electrons are the electrons in the highest occupied energy level of an atom. Mg2 Draw the Lewis dot structure for Mg2.
Please help me i dont know how to do this. Sorry for my horrible writing The structure should have single bond between Mg with the two bromides each contributing one electron. Apply the following guidelines in deciding what.
Write the electron configuration orbital notation of phosphorus atom and phosphorus ion. If you mean Mg2 it has no valence electrons so there is not much of a Lewis dot structure to draw. What is the lewis dot structure for Fluoride F1- and Mg2.
To change the symbol of an atom double-click on the atom and enter the letter of the new atom. Show the formal charges of all atoms in the correct structure. This is the MO diagram for the NEUTRAL Mg2 dimer.
The Lewis dot structure for Sulfur is an S with 6 dots which stand for its six valence electrons. Draw the Lewis dot structure for Mg2.
Solved They Dont Want A Picture Of The Lewis Diagram They Chegg Com

Write Lewis Dot Symbols For The Following Atoms And Ions I O Ii O2 Iii Mg2 Iv P3 V Br Snapsolve
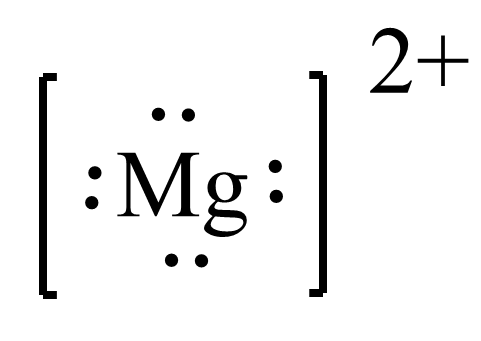
Answered Enter A Lewis Structure For Each Of The Bartleby

Mg 2 Electron Configuration Magnesium Ion Youtube

Electron Configuration And Lewis Dot Diagrams Ppt Download

Write Lewis Dot Structure Of Mg2 Brainly In

Download How To Draw The Mg2 Lewis Dot Structure In Mp4 And 3gp Codedwap
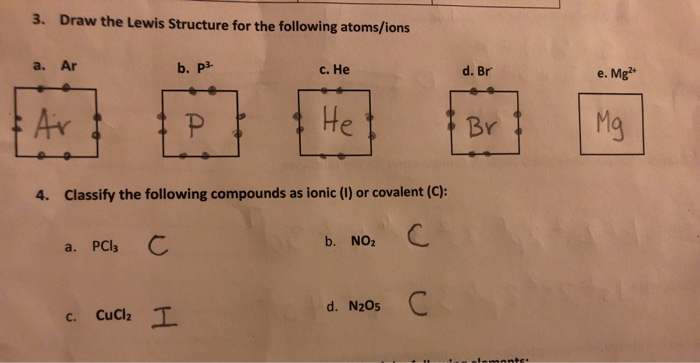
Solved 3 Draw The Lewis Structure For The Following Chegg Com
0 comments
Post a Comment